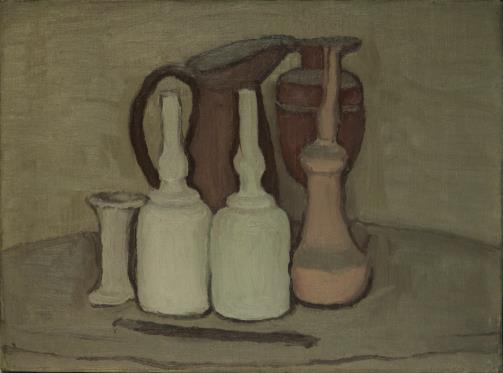In the midsummer, the new exhibition "Zhai Zi China" of Shanghai Museum, which was officially exhibited today, brought the audience to the Central Plains of Xia, Shang and Zhou Dynasties to explore the spirit and charm of the magnificent civilization in the pre-Qin period; "Tarrasa: Marine Civilization and Greek Art" makes people feel the wind blowing by the Aegean Sea. China National Museum "Oriental Lucky Gold" focuses on the historical culture, etiquette civilization, science and technology and artistic achievements of China, South Korea and Japan with ancient bronzes.
Overseas, the self-portrait discovered not long ago after Van Gogh’s "The Head of a Peasant Woman" was exhibited for the first time in the exhibition "Taste Impressionism: Modern French Art from millet to Matisse" at the Scottish National Gallery.
"The Paper Art Review" specially sorts out the exhibitions worth watching for a week, in order to provide dinner for readers.
Shanghai
House China —— Exhibition of Three Generations of Civilization in Xia, Shang and Zhou Dynasties in Henan Province
Venue: Shanghai Museum
Exhibition period: July 30-October 23, 2022

This exhibition is the first special exhibition of Shanghai Museum’s "Why China" cultural relics and archaeology exhibition series. Through more than 200 pieces (sets) of precious cultural relics from Xia, Shang and Zhou Dynasties, it leads the audience to trace back to the Central Plains in Xia, Shang and Zhou Dynasties and explore the magnificent spirit and charm of the pre-Qin period. The exhibition name "Zhai Zi China" comes from the inscription of He Zun, a bronze ware in the early Western Zhou Dynasty, and it is also the origin of the earliest known word "China".
Tarrasa: Marine Civilization and Greek Art
Venue: Shanghai Museum
Exhibition period: July 19-October 9, 2022

"Tarrasa" is taken from Thalassa, the goddess of the sea in ancient Greek mythology, which symbolizes the ocean that surrounds the Greek mainland and breeds many islands. It not only represents the main geographical features of Greece, but also is an important part of Greek life and psychology.
The exhibition displays 34 pieces (sets) of modern and contemporary Greek art works, and reproduces 5 ancient Greek cultural relics in the form of holographic projection, trying to explore and show the special relationship between Greece and the ocean from a historical perspective, and how they reflect the rich connotation of the ocean in visual art.
Prosperity and Prosperity —— Shanghai Museum’s Gifted Cultural Relics Exhibition
Venue: Shanghai Museum
Exhibition period: March 10th, 2022-January 8th, 2023.

There are 195 donated cultural relics in the exhibition, including Wang Nanping and Fang Shuyan’s "The Outline of Lengyan Sutra" donated by Wang Anshi in the Northern Song Dynasty, Fu Cha, the King of Wu in the late Spring and Autumn Period donated by Stanley Ho, and the Ming Dynasty Shen Zhou’s "Drawing the Axis of Xie ‘an Dongshan" donated by Weng Wange. The exhibition is another exhibition of cultural relics donated by Shanghai Expo after the "Prosperous Millennium" and "Mountain Scenery" held last year, and it is also a wonderful chapter in the celebration of the 70th anniversary of the Shanghai Museum this year.
Giorgio Morandi
Venue: Shanghai Jiushi Art Museum
Exhibition period: July 9-October 9, 2022
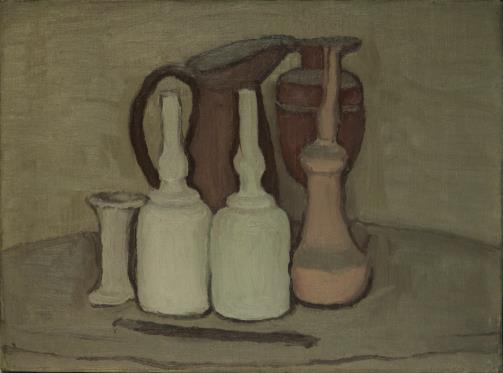
There are 51 original paintings of Mo Landi on display, including 39 oil paintings, 6 original prints, 4 watercolors and 2 sketches, spanning Mo Landi’s nearly 50-year creative career. The works on display are from the Mo Landi Museum in Bologna, Mo Landi’s hometown, the National Museum of Modern Art in Rome, the world-famous Giovannadi private collection and IMAGO Gallery.
Endless with Heaven —— Shaanxi Zhou, Qin, Han and Tang Cultural Relics Exhibition
Venue: Fengxian Museum, Shanghai
Exhibition period: July 15th-September 15th, 2022.

The exhibition, in chronological order, is divided into four units, namely, "Brilliant History of Zhou Dynasty", "Oriental Empire", "Heroes of Han Dynasty" and "Meteorology of Prosperous Tang Dynasty", which shows the splendid history of the Chinese nation and its energetic and tolerant cultural self-confidence from the aspects of political system, social economy, people’s livelihood culture and foreign exchange.
Dahan Haijiang: South Vietnam Maritime Civilization Exhibition
Venue: China Maritime Museum
Exhibition period: July 1-September 18, 2022.

154 pieces/sets of unearthed cultural relics were exhibited, including the only complete silk jade garment in the world, the largest gold seal in the Western Han Dynasty, and the only dragon button emperor seal in the Han Dynasty, which showed the unique Lingnan style and maritime culture of South Vietnam.
Straight and detour: the path of modern architecture in Taiwan, China
Venue: Shanghai Museum of Contemporary Art
Exhibition period: July 16th-October 16th, 2022.

The exhibition will introduce nearly 60 works by 16 architects/studios in Taiwan, China, and display related manuscripts, films, paintings, photographs, documents, models and immersive experience devices. The exhibition aims to sort out the development and latest exploration of modern architecture in Taiwan Province, and show the architect’s prudence and reflection on its identity.
Transforming the Future-The 8th Shanghai Duolun Youth Art Exhibition
Venue: Shanghai Duolun Modern Art Museum
Exhibition period: August 2-August 31, 2022

In the exhibition, works were collected by public solicitation and nomination and recommendation, and finally the works of 18 artists/groups were selected by the expert judges, covering painting, installation, sound, video and performance.
Beauty of Power —— Lu Xun and Printmaking
Venue: Shanghai Luxun Memorial Hall
Exhibition period: July 26th-September 12th, 2022.

The exhibition displays 89 pieces (sets) of rubbings, notes, Japanese ukiyo-e, European prints and prints created by China emerging woodcut artists trained by Lu Xun, among which 11 pieces are first-class cultural relics, and most of the rest are second-class cultural relics, in addition to 15 works of art by famous artists. In this way, it presents the accumulation process of Lu Xun’s artistic thoughts, and advocates and promotes the artistic creation of modern woodcut prints in China.
Long-term design: thinking and practice
Venue: Pearl Art Museum
Exhibition period: July 3-November 6, 2022

With more than 600 design works in 9 units, the exhibition goes deep into the traditional and modern features of regional products, and comprehensively and systematically introduces the design thinking and multi-field practice of Nagaoka Sage.
Jiangnan Life Aesthetics Exhibition
Venue: Shanghai Museum of History
Exhibition period: July 29th to August 28th, 2022.

Taking cultural relics as the carrier, the exhibition aims to show Jiangnan culture which is connected with rich, poetic, picturesque, moral and other cultural meanings. At the same time, the "Green Gold Beautiful Life-Fantasy Tour of China Tea in 17th-19th Century" presents the maritime tea trade in 17th-19th century and the influence of China tea culture on the world. This exhibition can be regarded as an extension of "life in the south of the Yangtze River", and can be viewed in contrast.
Nara Yoshitomo
Venue: BudiTek Art Museum (temporarily closed)
Exhibition period: March 5-September 4, 2022

Through more than 70 important paintings, sculptures, ceramics and installations, and more than 700 manuscripts that have not been exhibited, the exhibition reviews Nara Yoshitomo’s prolific artistic career spanning 37 years. This exhibition is not only the first solo exhibition of Japanese artist Nara Yoshitomo in Chinese mainland, but also his first large-scale retrospective tour around the world.
Water Domain —— Exhibition of the Kebranly Museum and the Fran? ois Schneider Foundation in France
Venue: Pudong Art Museum
Exhibition period: until August 31st.

The theme of "Water Domain" is water, which is the core of human life. It presents more than 100 collections of exhibits from the French Kebranli Museum and the Fran? ois Schneider Foundation, and has a unique dialogue from five aspects: water landscape manufacturing, water daily life, liquid imagination, sacred territory and water geographical transition.
Thomas Dimander: The Tongue of History
Venue: UCCA Edge
Exhibition period: July 8-September 4, 2022

Through more than 70 works such as photography, movies and wallpaper created by the artist in his career, the exhibition systematically sorts out the artistic practice of integrating sculpture and photography and exploring the junction of history, images and architectural forms.
The voice of all things
Venue: West Coast Art Museum (temporarily closed)
Exhibition period: July 28th, 2021-February 5th, 2023.

The exhibition brings together more than 160 masterpieces collected by Pompidou Center, and leads the audience into the hinterland of the avant-garde art movement under the background of globalization from the beginning of the 20th century to recent years with 18 exhibition chapters, touching the pulse of historical times with "things" and sinking into the sound behind listening to art..
Never Here —— Exhibition of Picasso, Van Donggen and Wu Lintian’s Works
Venue: Jane Gallery (CJ Fine Art, No.1, Lane 800, Zhongshan South Road)
Exhibition period: August 5-September 29, 2022


The exhibition combines eight Picasso’s works, two illustrations created by Van Dongen for Kipling’s collection of short stories, The Most Beautiful Story of Kipling, a famous British writer, and seven works recently created by China artist Wu Lintian, showing a special collision and integration of Chinese and Western arts. The Picasso series prints exhibited this time are the last set of prints made by Picasso before his death. In 1969, Picasso, who was 88 years old, spent two months creating 29 delightful portraits on corrugated paper with watercolor pigments, including mustaches, musketeers, deconstructed female faces, and abstract faces of Balzac, Shakespeare, Rembrandt and other literary giants. Among them, "Musketeer" series is one of the most important creative themes of the artist in his later years.
Wu Lintian is a painter who is engaged in abstract art creation and studies the tradition of Chinese painting. The seven works in this exhibition have changed their previous fascination with black and brown, and are mainly light pink. The works take the ancient towns, villages, pastoral areas and streets around the artist’s studio as the main reference objects, and complete the imagery creation by means of board linen and homemade pigments.
Substitute flowers for trees-Betan Lavier
Venue: Shanghai Fosun Art Center
Exhibition period: July 1st-September 4th, 2022.

This is the 73-year-old French national treasure artist’s first exhibition in Chinese mainland. Looking back on its pioneering artistic practice, the exhibition explores the relationship between painting and sculpture, expression and abstraction, and life and art with its iconic misappropriation art, and at the same time, creates a fantastic world of subversion for the audience through the concept of "scene construction".
Huayuan-Natural Return to the City
Location: moca shanghai.
Extension: lasting until mid-August.

With the unique curatorial concept of "thinking", the exhibition focuses on introducing the topic of "supernatural city". It is composed of three exhibition areas and seven unit theme exhibitions, bringing together the works of 12 artists, covering paintings, installations, images, photography and other media, and expressing the topic of "sustainable development" with the continuous increase of temperature in a cool way.
Your childhood, my memory —— Children’s images in contemporary photography
Venue: Biyun Art Museum, Pudong
Extension: lasting until September 18th, 2022.

Through 10 groups of 99 works by many artists, the exhibition shows some concrete achievements in children’s image creation in China since the reform and opening up, including both documentary works reflecting social changes and childhood scenes "imagined" thanks to digital technology.
Beijing
Worship Wei Yao De —— Qing Dynasty Military Equipment Exhibition in the Palace Museum
Venue: Beijing Guardian Art Center
Exhibition period: July 22-October 30, 2022

According to their functions, the exhibition divides the Qing Palace’s armament cultural relics into three units, namely "Courtesy to Heaven and Earth", "Shenfeng Grasping Victory" and "Baoye Ningtao", showing the application of armament in etiquette and war and its artistic characteristics.
Encounter Mesopotamia: Fine Exhibition of Ancient Syrian Cultural Relics
Venue: National Museum of Classics
Exhibition period: June 25th-September 12th, 2022.

The area where the Tigris River and the Euphrates River, which originated in Anatolia mountains in history, flow is called the two-river basin, and the ancient Greeks called it Mesopotamia, which means "the land between the two rivers". The exhibition displays 196 pieces/groups of exquisite cultural relics from Syria, depicting a long historical and cultural picture, and also showing the friendly cultural exchanges between China and Syria.
The Origin of Italy-Exhibition of Ancient Roman Civilization
Venue: National Museum
Exhibition period: July 10th-October 9th, 2022.

The exhibition gathered 503 ancient Roman cultural relics to tell the lifestyle, folk customs and social reality at that time.
Oriental Lucky Gold-Exhibition of Ancient Bronzes in China, Korea and Japan
Venue: National Museum
Exhibition period: July 26th-October 9th, 2022.

About 50 pieces (groups) of fine bronze cultural relics collected by the national museums of China, South Korea and Japan are displayed in the exhibition, taking bronze as the carrier, presenting the ancient history and culture, etiquette civilization, science and technology and artistic achievements of the three countries.
Accumulate Thick and Spread Wide —— Exhibition of Archaeological Achievements of National Museum
Venue: National Museum
Extension: from July 2, 2022.

In the exhibition, more than 240 representative archaeological relics were selected from different dimensions of land, sea and air to show the archaeological achievements of Guobo around specific archaeological cases.
Rong Yao Dan Qing —— Portrait Exhibition of Ming and Qing Dynasties in China National Museum
Venue: National Museum
Exhibition period: from April 29th, 2022.

The exhibition consists of three units, and more than 50 masterpieces (sets) of portraits of Ming and Qing dynasties are selected. The themes cover the royal family, famous officials, celebrities, literary societies, boudoir, women’s faces and other aspects, and the interpretation of the functional meaning of paintings is systematic, diverse and in-depth. Including "Sitting in Kangxi’s Study", "Du Fu’s Herb-picking Map" and "Chasing Huan and Getting Lu Map".
Sven Chuan Gu Feng: Deng Tuo donated ancient painting exhibition.
Venue: China Art Museum
Exhibition period: June 21st-August 25th, 2022.

To commemorate the 110th anniversary of Deng Tuo’s birth, China Art Museum held this exhibition, displaying more than 70 paintings donated by Deng Tuo. Through the three parts of "Xiaoxiao Bamboo, Lei Leishi", "Moving Vivid Quality to Freehand Style" and "Seeing the Road with Sincerity", the cultural value and charm of China’s ancient paintings were interpreted. The exhibition will also present a set of photos, documents and books for the first time, showing Deng Tuo’s elegance and charm.
A Master and a Half-bosom Friend —— A New Situation of Flower and Bird Painting by Qi Baishi, Yu Feian and Wang Xuetao
Venue: Beijing Painting Academy
Exhibition period: June 30th-September 18th, 2022.

The exhibition collected more than 70 pieces of flower-and-bird paintings by Qi Baishi, Yu Feian and Wang Xuetao. From the perspective of artistic inheritance, the exhibition studied three different types of flower-and-bird painters as a whole, and analyzed and explored the fundamental reasons why modern flower-and-bird painters opened up a "new realm" in the era from the four dimensions of "tracing back to the ancients", "imitating the ancients" and "expressing their feelings" for the broad audience.
Architecture with Five Senses: Kengo Kuma Architectural Design Exhibition
Venue: Guardian Art Center
Exhibition period: July 22-October 30, 2022.

The exhibition starts from the "five senses" from the presentation to the content, emphasizing all the senses of the future architecture responding to the lawsuit, and exhibiting works such as Bamboo Stream, Bamboo Qu, Bamboo and Stone, which bring comfort to people’s hearts.
Peking University Archaeology 100 Years Special Exhibition of Archaeology Major in 70 Years
Venue: Secler Museum of Archaeology and Art, Peking University
Exhibition period: May 3, 2022-May 10, 2023

The exhibition presents more than 100 pieces (sets) of exhibits, including cultural relics specimens, excavation reports, photos and literature files excavated by Peking University Archaeology over the years. There are two exhibition lines inside and outside the exhibition. The inner line is the memorabilia of the college, and the outer line introduces the important archaeological work that Peking University participated in and its contribution to the development of discipline theory and method.
Zhejiang(Province)
Wind and Lotus —— Lotus Culture and West Lake in Hangzhou
Venue: West Lake Art Museum
Extension: lasting until September 7th.

There are 170 lotus-themed cultural relics and handicrafts on display in the exhibition from Neolithic Age to modern times, all of which reveal the charm and elegance of lotus-themed cultural relics, from the Chicken Head Pot in the Six Dynasties, the celadon in Yueyao, the celadon with lotus patterns in Longquan Kiln in the Song and Yuan Dynasties, to Chen Hongshou’s "Lotus with Two Butterflies".
Metaphysics —— Longquan celadon from the perspective of Song Yun
Venue: Zhejiang Provincial Museum
Exhibition period: June 18th-August 18th, 2022.

More than 20 pieces of southern song Longquan celadon unearthed from the cellar in Jinyucun Village, Sichuan Song Porcelain Museum were exhibited, including the southern song Longquan celadon bottle, the southern song Longquan celadon water purification bowl and the southern song Longquan celadon ear-piercing bottle. This is also the second series exhibition of Longquan celadon with the theme of comparison between ancient and modern after 2021.
Antique Shadow —— Huang Binhong’s Ancient Seal Collection Exhibition
Venue: Zhejiang Provincial Museum (Wulin Hall Area)
Exhibition period: June 1st-September 3rd, 2022.

In the exhibition, 129 buttons of ancient seal collected by Huang Binhong before his death are displayed in contrast with his calligraphy and interpretation, supplemented by more than 40 manuscripts of seal science and ancient philology and paintings and calligraphy works. Through Huang Binhong’s collection, this paper aims to explore his views on collection, art and values, so as to pursue the interaction between Huang Binhong’s artistic creation and collection and his hidden spiritual pursuit.
Ding Bing and Hangzhou in the 19th Century
Venue: Hangzhou Museum
Exhibition period: May 18th-November 20th, 2022.

The exhibition is the first exhibition of the "Qiantang Tide" urban culture series, with a total of 63 sets/pieces, including letters, scrolls, rare books, bronzes, silks, ceramics, furniture and many other categories. The exhibition is divided into three units, namely "People on Qiantang River", "Reviving the Old Lakes and Mountains" and "Relieving Hang Cheng". It focuses on the construction and reform of Hangzhou gentry represented by Ding Bing in the late 19th century in many fields, such as industry, education, culture, charity and city, which provides a reference for China’s modernization.
From Hutemas to Future Vision: Design History of Soviet Russia —— Creating a New World
Venue: China International Design Museum of China Academy of Fine Arts.
Exhibition period: March 17th-September 22nd, 2022.

Focusing on the design innovation from Soviet Russia to the Soviet Union, the exhibition is the first large-scale exhibition of the complete design history of this period in China, aiming at exploring the pioneering experiments and achievements of the design in this period for building a new life world, and tracing back to the endogenous motivation and creativity in the design and art fields. The types of works presented in the exhibition cover drama, architecture and handicrafts related to this theme, as well as most modern design categories such as products, planes, clothing and transportation.
Jiangsu(Province)
"Jin State" Special Exhibition
Venue: Nanjing Museum
Exhibition period: June 25th-October 7th, 2022.

The exhibition displays 231 pieces (sets) of fine cultural relics from Shanxi Museum. Through three units, namely "Favourite of Hefen", "Striving for hegemony in the Spring and Autumn Period" and "Yu Lie San Jin", it focuses on the 600-year great achievements of the State of Jin from "sealing brothers with tung leaves" to "dividing Jin into three families".
Pan Yuliang painting special exhibition
Venue: Nanjing Museum
Exhibition period: July 16th-October 10th, 2022.

The unique artistic interest of Pan Yuliang’s works under the collision of Chinese and western cultures is realized from three units: Portrait and Figures, Women and Human Body, Scenery and Still Life.
"Enriching the Brilliance, Being Beautiful and Being Great" Series Exhibition to Commemorate the 100th Anniversary of Suzhou Art College.
Venue: Suzhou Art Museum and Yan Wenliang Memorial Hall.
Exhibition period: from July 15th.

The exhibitions include "Picturesque Canglang Overlooking the South of the Yangtze River-Invitation Exhibition of National Oil Paintings by Famous Oil Painters" (Suzhou Art Museum), "Looking at Canglang Festival-Yan Wenliang and Suzhou Academy of Fine Arts, Canglang’s Three Masters’ Eyes and Beads"-Exhibition of the Works of Yan Wenliang, Hu Cuizhong and Zhu Shijie, the main founders, and the exhibition of the works of Wu Zishen, an important sponsor, etc.
The Appearance of Civilization —— Exhibition of Ancient Cultural Relics in Eurasia
Venue: Suzhou Wu Culture Museum
Exhibition period: June 30th-September 20th, 2022.

The exhibition brought together more than 190 sets (pieces) of representative cultural relics of various civilizations on the Eurasian continent, and the cultural relics were collected from Syria, Afghanistan, Iran, Turkey, Italy and other countries. These ancient cultural relics from foreign countries show some characteristics of the formation and development of civilizations in the Mediterranean region, western Asia and central Asia, and lead the audience to feel the characteristics of cultures in Asia and Europe and the exquisiteness of cultural relics from different angles.
Beauty surnamed Dong
Venue: Suzhou Museum
Exhibition period: June 3-August 31, 2022

The exhibition found 10 "Dong Shi" recorded in history, from Dong Meiren in Sui Dynasty to Dong Xiaoyuan in late Ming Dynasty, including more than 70 pieces (sets) in different categories, including gold and silver wares, porcelain, inscriptions, ancient books, calligraphy and painting, showing the beauty of ancient ladies’ daily life, telling the marriage and life, talents and images, feelings and ideals of women in Dong Shi through one piece of cultural relics, and using this as a window to explore China.
Zhongzhi Shenzhou —— Gorgeous Luoyang City in Tang Dynasty
Venue: Yangzhou China Grand Canal Museum
Exhibition period: May 27th-August 28th, 2022

There are more than 230 exhibits (groups) in this exhibition, which are from the Institute of Archaeology of Chinese Academy of Social Sciences, Longmen Grottoes Research Institute, Luoyang Museum, Luoyang Institute of Cultural Relics and Archaeology and shangcheng museum in yanshi. The exhibits are all archaeological excavations and collections of Tang Dynasty remains in Luoyang, among which there are rich first-class cultural relics, many of which are exhibited for the first time.
Purple jade is full of fragrance —— Special exhibition of Yixing purple sand in Nanjing Museum
Venue: Yangzhou Museum
Exhibition period: July 29th-October 9th, 2022.

92 pieces/set of Yixing purple sand ware were displayed, and the traditional craft, interesting aesthetics and literati charm of Yixing purple sand were shown to the audience from three aspects: "purple jade and gold sand", "the music is wonderful" and "the rhyme is full and dry".
Henan(Province)
Wang Duo Calligraphy Art Exhibition —— With an array of horses and a pen.
Venue: Henan Museum
Exhibition period: July 8-September 12, 2022

The exhibition carefully selected nearly 80 pieces (sets) of exhibits from 20 cultural and cultural institutions, covering the representative calligraphy works and rubbings handed down from ancient times in Wang Duo, as well as some works of making friends. They were gathered together and displayed in four parts, namely, "Imitating the Sage from Ancient Times", "Exploring the Path to Find Yourself", "Being Broad-minded" and "Writing by Literary Friends", which not only reflected the specific style and aesthetic orientation of the times.
Hebei
Fulu Shouxi Good Life —— Special Exhibition of Auspicious Culture in China
Venue: Hebei Museum
Exhibition period: July 8-November 8, 2022

More than 300 pieces (sets) of exhibits with auspicious meanings have been carefully selected in this exhibition, and the exhibition theme of "Fu Lu Shou Xi" has been condensed, including porcelain, jade, gold and silver wares, calligraphy and painting, fabric, paper-cutting, animal specimens, scientific painting, etc. Through the excavation of traditional auspicious culture in China, the audience can deeply understand the natural and humanistic attributes of animals and plants, and the important influence of auspicious culture on national spirit and social life.
Gracefulness —— The Elegant Life of Empresses in Qing Dynasty
Venue: Chengde Museum
Exhibition period: July 25th-October 25th, 2022.

The theme of the exhibition is the life of imperial concubines in Qing Dynasty. Through three units, namely "beautiful clothes and ornaments", "famous utensils" and "leisure and elegance", nearly 80 cultural relics, such as dress accessories, makeup combs, literary study, calligraphy and painting, needlework embroidery and tea set tableware, are displayed to reproduce the graceful, quiet, rich and unique court life of imperial concubines in Qing Dynasty.
Hunan
Infinite Beauty —— Literature and Female Images in Figure Paintings in Ming and Qing Dynasties
Venue: Hunan Provincial Museum
Exhibition period: June 23rd, 2022-October 30th.

The exhibition is divided into four units: the first unit expresses ancient women’s contribution to the family, the second unit shows the realization of ancient women’s social values, the third unit conveys the influence of women’s spiritual strength on the world, and the fourth unit explains the awakening of women’s self-awareness, focusing on the multiple social roles played by ancient women, their self-cultivation and sense of home country, and showing the literature and female images in figure paintings in Ming and Qing Dynasties.
Shanxi
The Great Man and the King of Chu —— Xuzhou Han Dynasty Chu Cultural Relics Exhibition
Venue: Shanxi Museum
Exhibition period: June 21st-October 10th, 2022.

More than 270 pieces of Han Dynasty cultural relics selected in the exhibition are mainly unearthed from the tombs of several Han Dynasty princes in Xuzhou, supplemented by the tombs of the emperors in Shanxi and related Han tombs, which interpret the historical features of the Han Dynasty vassal countries from various angles and provide a new perspective for the public to understand the culture of the Han Dynasty.
Guangdong
Faxian Dingyao
Venue: Nanyue King Museum
Exhibition period: June 21st-September 20th, 2022.

In the exhibition, 294 pieces/sets of porcelain, kiln furniture and specimens from Ding Kiln in various periods collected by many cultural and cultural institutions in Hebei and Nanyue King Museum were exhibited, including 22 pieces/sets of first-class cultural relics. Combined with exquisite cultural relics, it is divided into six themes: Famous Dingzhou, Beyond the Dust for a Thousand Years, Gunnar Palace, Elegant Geometry, Great White in the World and Millennium Covenant. Through the history, decoration, technology, culture and communication of Ding Kiln, the product appearance, cultural connotation and the development of contemporary Ding porcelain in different periods are presented.
Hong Kong
Opening Exhibition of Hong Kong Palace Museum of Culture
Venue: Hong Kong Palace Museum of Culture
Extension: from July 2, 2022.

Seven special exhibitions were launched in the first phase, presenting more than 700 precious cultural relics from the Palace Museum in Beijing, covering 25 categories such as paintings, calligraphy, bronzes, ceramics, gold and silver wares, jade articles, lacquerware, sculptures, books and classics, with a time span of 5,000 years.
Taipei
The quintessence of rare books and ancient books collected in the Academy —— Brahman and Classic Fold
Venue: North Campus of National Palace Museum in Taipei
Exhibition period: until September 11th.

The exhibition is divided into two parts: "Brahma" and "Sutra". The former starts with "books written on leaves", selects and displays the sutra of Bayleaf in Myanmar, and tells the origin of "Brahma". In the latter, books are hand-rolled, and the earliest Chinese tripitaka-Chongning Zang (in the early 12th century) is matched. After comparing and expounding its evolution, various classics such as Confucianism, Buddhism and Taoism are displayed, showing its unique style of solemnity, prudence, simplicity and elegance.
Popular national treasure exhibition
Venue: Southern Campus of National Palace Museum in Taipei
Exhibition period: January 25th, 2022-January 29th, 2023 (the exhibits will be updated on July 26th).

Focusing on the famous works in the art history collected by the National Palace Museum in Taipei, the cultural relics on display are all popular national treasures. On July 26th, the masterpieces of the Qing Dynasty, such as Yu Bi Shu Wan Shi Shi Shi Biao and fleshy stones, will be exhibited, and Wang Xizhi’s Three Postings of Peace, How to Serve an Orange, will be exhibited from October 4th to October 30th.
Worm hide and seek
Venue: North Campus of National Palace Museum in Taipei
Extension: lasting until September 25th.

The exhibition focuses on the paintings of grass insects in past dynasties, hoping that the audience can find out these little creatures captured by the painter, know their true identities, and understand the stories related to grass insects and the hidden meanings behind them.
abroad
Britain
Taste Impressionism: French Modern Art from millet to Matisse
Venue: National Gallery of Scotland
Exhibition period: July 30th-November 13th, 2022.

The exhibition shows many world-class masterpieces such as Van Gogh, Degas and Gauguin, as well as seven complete sets of Jazz by Monet and Matisse, which tells how Scotland became one of the greatest collections of impressionist and post-impressionist art in the world. The self-portrait just discovered after Van Gogh’s "The Head of a Peasant Woman" in 1885 is also displayed in the form of a light box.
Anatomy: about life and death
Venue: National Museum of Scotland
Exhibition period: July 2-October 30, 2022
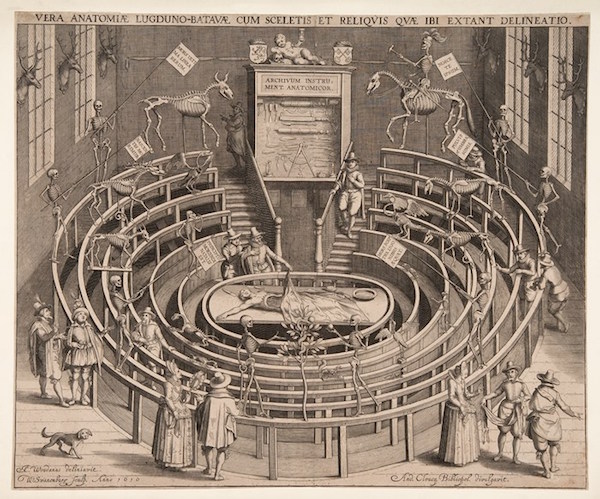
The exhibition explores people’s pursuit of anatomical science in the past 500 years, and also shows the art and history related to it. These include Leonardo da Vinci’s sketches and the bones of the corpse trafficker William Burke, a man who killed for science and eventually became a scientific specimen himself.
Pre-Raphael School: Sketch and Watercolor
Venue: Ashmoline Museum, Oxford
Exhibition period: July 15th-November 27th, 2022.

More than 100 pre-Raphael works on paper (including portraits created by painters, commissioned paintings, and themes such as history, literature, and scenery) were exhibited. Most of the works are collected by the Ashmoline Museum since 1893, mainly from the legacy of Martha Qom, a pre-Raphael patron.
Tutankhamun: Digging Files
Venue: Bodley Library, Oxford University
Extension: lasting until February 5, 2023.

This year marks the centenary of the discovery of Tutankhamun’s tomb by British archaeologist howard carter and his team. Different from previous exhibitions which focused on Tutankhamun and howard carter, this exhibition paid special attention to the contributions of other members of the team outside Carter, especially the unknown Egyptian workers who helped to discover Tutankhamun’s mausoleum.
Milton Avery: American master of color matching
Venue: Royal Institute of Art, UK
Exhibition period: July 15th-October 16th, 2022.

Milton Avery (1885 -1965), a modern American artist, is regarded as one of the greatest color masters in North America in the 20th century. He described the American land and seascape in the wild way of French Fauvism, which had an important influence on the creation of Roscoe, Pollock and others. The exhibition presents 70 paintings created by him from 1930s to 1960s, which are usually characterized by depicting daily life, including portraits of relatives, tranquil scenery in Maine and Cape Cod.
Edvard munch: A masterpiece from Bergen.
Venue: Courtyard Gallery
Exhibition period: May 27th-September 4th, 2022.

From the 1980s to 1909, the exhibition spanned several decades, and only 18 works of Monk were exhibited, but each one was a masterpiece full of vitality and dramatic tragedy. The works on display include Morning (1884), Summer Night (1889), Night of Karl John (1892), Melancholy (1894-1896) and Before Dying (1895), which shows the development of Munch’s art.
Picasso and Angel: Face to face
Venue: British National Museum
Exhibition period: June 3-October 9, 2022

Picasso’s Woman with a Book (1932) at the Norton Simon Museum in California was exhibited for the first time alongside its inspiration Anger’s painting Madame Moisil. Picasso met the mysterious Madame Moisil for the first time in an exhibition in Paris in 1921, and was deeply fascinated. In the next ten years, Picasso repeatedly paid tribute to Angel in his works of art.
Female Power: From Gods to Demons
Venue: British Museum
Exhibition period: May 19th-September 25th, 2022.

With the theme of "Women’s Power", the exhibition brings together ancient paintings, sculptures, sacrificial objects and modern and contemporary artworks from six continents around the world, focusing on the female gods and demons worshipped and feared by human beings in the past 5,000 years, and examining the role played by women in shaping human cognition of the world from a cross-cultural perspective.
Reconstruction: the woman in the window
Venue: Dulwich Art Museum, London
Exhibition period: May 4th-September 4th, 2022.

The exhibition spans all ages, starting from an ancient Greek vase, and brings together more than 50 works by Botticelli, Luo Saidi, Hockney: A Life In Pictures, Bourgeois, Cindy Sherman and other artists, revealing how artists interpret women in different times and cultures with a traditional theme.
United States of America
Chrome: Ancient Color Sculpture
Venue: Metropolitan Museum of Art, New York
Exhibition period: July 5, 2022-March 26, 2023

In the original and energetic form, the exhibition "restored" and reproduced 14 ancient Greek and Roman statues such as the Sphinx, as well as more than 40 other ancient sculptures and pottery. In the bright exhibition hall of the Metropolitan Museum, they talk with ancient sculptures polished by time into pure white.
Memory of water
Venue: Metropolitan Museum of Art, New York
Extension: lasting until April 2, 2023.

This is a small, poetic and multifaceted exhibition, which discusses the material and symbolic role of water in Native American life. Organized by Patricia Marroquin Norby, deputy curator of American art at the Metropolitan Museum of Art, the exhibition combines traditional cultural relics in the museum’s permanent collection with contemporary works on loan, some of which are made by non-indigenous artists.
Raphael-The Energy of Renaissance Images: Dresden Tapestry and Its Influence
Venue: Columbus Museum of Art, USA
Exhibition period: it will be exhibited until October 30, 2022.

Six large-scale tapestries of biblical scenes designed by Raphael were exhibited, which were based on the same batch of design sketches as Raphael’s tapestries for the Sistine Chapel, and were woven by a workshop in London after Raphael’s death. The exhibition tries to show the power of image reproduction hundreds of years ago and the influence of Raphael tapestry painting on later artists.
"Cezanne" Special Exhibition
Venue: Chicago Museum of Art
Exhibition period: May 15th-September 5th, 2022.

The exhibition explores Cezanne’s cross-media and cross-genre artistic practice through 80 oil paintings, 40 watercolors and sketches, and two complete sketches. In addition to still life, portrait theme, impressionist landscape painting, Mount St. Victor, "Bather" series and other iconic painting themes of Cezanne, the exhibition also exhibited early fable paintings that were little known. These works combine the artist’s palette, composition deconstruction and other most advanced technical analysis to interpret how Cezanne conceived and made the image step by step towards the truth of the human eye.
Matisse: The Red Studio
Venue: new york Museum of Modern Art (MoMA)
Exhibition period: May 1st-September 10th, 2022.

The exhibition brings together six existing paintings, three sculptures and a ceramic work from the works in The Red Studio, and presents them in the exhibition-they were in Matisse’s studio when Matisse drew The Red Studio. It is reported that this is the first reunion of these works after more than one hundred years.
Diego Rivera’s America
Venue: San Francisco Museum of Modern Art
Exhibition period: July 16th, 2022-January 2nd, 2023.

The exhibition presents 150 oil paintings, murals and sketches by this Mexican artist. The exhibition focuses on Rivera’s creation from the 1920s to the 1940s, when he regarded murals as "weapons" of social change and expressed his vision of "Pan-American reunification" on both sides of the border between Mexico and the United States.
Georgia O ‘Keeffe, photographer.
Venue: Denver Art Museum, USA
Exhibition period: July 3-November 6, 2022

When Ou Jifu devoted herself to photography in the mid-1950s, her creative identity and unique artistry had been established. Photography showed the artist’s persistent fascination with natural cycles and changes, while the exhibition was a tribute to Ou Jifu’s fascination with visual metaphors.
Norway
Norway’s new national museum opens
Venue: New National Museum of Norway
Exhibition period: from June 11th.

The new National Museum of Norway is composed of the National Gallery of Norway, the Museum of Architecture, the Museum of Decorative Arts and Design and the Museum of Contemporary Art, with a cost of about 500 million pounds and more than 6,500 collections on display. The new National Museum specially set up the "Monk Hall" for edvard munch, displaying 18 core works of Monk, including Scream.
Italy
The 59th Venice Biennale
Venue: Venice
Exhibition period: April 23rd-November 27th, 2022.

The theme of the exhibition is the short story "Milk of Dreams" by Leonora carrington, a British-born Mexican surrealist artist, which discusses post-human, deformation, ecology and other issues. The exhibition itself is like a revival of surrealism in this era.
The theme exhibition of this Biennale has 213 artists from 58 countries and 81 countries. It is reported that the number of artists participating in this Biennale will be the highest since 2005, which is double the number of the previous Biennale. At the same time, this Biennale has set the highest number of female artists, and more than 80% of the participating artists are women.
France
The Golden Age of the Portuguese Renaissance
Venue: Louvre
Exhibition period: until October 10, 2022.

About 15 Portuguese paintings that are rare in France are exhibited, showing this fragment that is rarely mentioned in the history of art. The Portuguese Renaissance combined Flemish style with local national traditions, and developed its own unique style by combining keen and even humorous observation and narration.
German/1920s/New Objectivism/august sander
Venue: Pompidou Art Center
Extension: lasting until September 5, 2022.

The exhibition covers painting, photography, architecture, design, literature and music, etc. From Santer’s photographic work "People in the 20th Century" to otto dix’s portrait series, it depicts a group image of the 20th century and resonates with the present world undergoing various changes.
Germany
The 15th Kassel Literature Exhibition
Location: Castle Land
Exhibition period: June 18th-September 25th, 2022.

The exhibition was curated by ruangrupa, an Indonesian artist group. They got inspiration from the "Micang" in rural Indonesia, formed the curatorial concept, and regarded the literature exhibition as a collective resource "from and used in Kassel city" to support local artistic practice.
The 12th Berlin Biennale
Venue: Berlin Art Institute, Hamburg Railway Station Art Museum, KW Center for Contemporary Art, etc.
Exhibition period: June 11th-September 18th, 2022.

The theme of the 12th Berlin Biennale is "Still exists!" Curator Kader attiya, a French-Algerian interdisciplinary artist, discussed the theme of "Crazy and destructive competition of global capitalism in production". Other topics covered in the exhibition include global warming, decolonization and the role of human beings in it. However, what supports this Berlin Biennale is neither the past nor the colloquial future, but it points to the real world in different ways.
Schliemann’s world
Venue: James Simon Art Museum and New Museum.
Extension: lasting until November 6, 2022.

2022 is the bicentennial of the birth of Heinrich Schliemann, a German archaeologist and discoverer of the ancient city of Troy. There are about 700 exhibits on display in this exhibition, including spectacular archaeological excavations, as well as many international borrowed exhibits, which will focus on the unexposed side of schliemann and what he was like before he started archaeology. The exhibition also uses the current research results to critically examine the archaeological methods of schliemann’s time.
Switzerland
The Evolution of mondriaan
Venue: Basel Beyeler Foundation
Exhibition period: June 5-October 9, 2022

There are 89 works on display, 82 of which are borrowed from art institutions and private collectors all over the world. Through the comparison and continuation of works in different periods, we can see the changes of mondriaan’s art.
Picasso-El greco
Venue: Basel Art Museum
Exhibition period: June 11th-September 25th, 2022.

The exhibition traces greco’s influence on Picasso in the form of a pair of their works, highlighting the lifelong kindness of the "old master" to the modernists.
Japan
In 2022, the wife of Yue Hou had an art festival of the earth.
Location: Yue Hou’s wife has
Exhibition period: April 29th-November 13th, 2022.

Among this year’s earth art festival, there are the dead Christian’s "Mori Jing", the Russian artist Kabakov and his wife’s new work "Tower of Holding Hands" that have participated since the first session, and the Ukrainian artist Jenna Kadyrova’s "stone bread" that yearns for daily life and peace; The works of Japanese local artists reflect the local culture and the use of daily tools.
Dialogue between Nature and Man: From Friedrich, Monet, Van Gogh to richter
Venue: National Western Art Museum
Exhibition period: June 4-September 11, 2022

To celebrate its reopening, the National Museum of Western Art in Japan launched this exhibition in cooperation with the Museum Folkwang, Essen) in Germany. Focusing on the "Dialogue between Nature and Man", it brought together more than 100 collections from German Romanticism, Impressionism and Post-Impressionism to modern art in the 20th century, and traced back the changes of western perception of nature and artistic expressions in modern times.





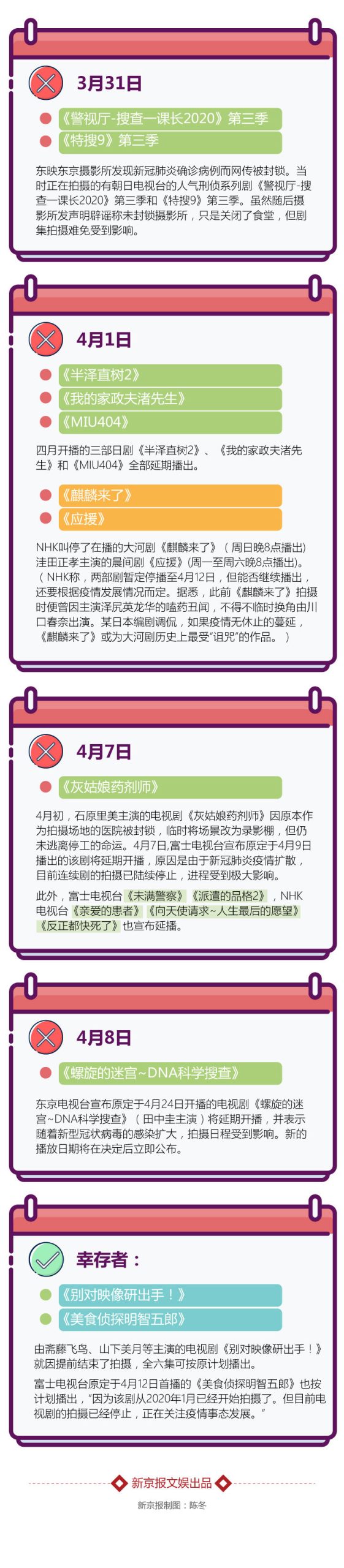 Shooting while broadcasting leads to "no inventory", avoiding kissing scenes to reduce the risk of infection.
Shooting while broadcasting leads to "no inventory", avoiding kissing scenes to reduce the risk of infection.